- 7 US healthcare systems integrated the new, touch-free, DiskCover® System for stethoscope hygiene.
- After a month of routine use, over 93% of participating clinicians believe the technology is superior to other options and improves patient safety.
- Clinicians also report The DiskCover System is easy to use, while improving clinical workflow and hygiene compliance.
SAN DIEGO, March 9, 2023 /PRNewswire/ -- Over the course of hundreds of patient examinations, scores of physicians, nurses, and other clinicians utilized a new, touch-free, aseptic answer to stethoscope contamination and found it superior on a variety of factors, including patient safety, to other available options, according to a newly published clinical study in the International Journal of Health Policy and Planning, titled "Stethoscope Hygiene, Workflow, and Patient Safety: The Crux of Healthcare Associated Infections." The technology, developed by San Diego start-up, AseptiScope®, was designed, patented, and validated to resolve a problem that has been undermining infection control efforts in hospitals for over a hundred years: stethoscope contamination. Alcohol cleaning takes time and skill, destroys the stethoscope diaphragm, and is becoming less and less effective against all pathogens, including highly resistant organisms. Disposable stethoscopes, another common option for stethoscope hygiene, compromise auscultation quality and can result in harmful misdiagnoses. The new DiskCover System instantly provides an aseptic, acoustically invisible barrier that is significantly more effective than alcohol cleaning. Equally impressive, this automated technology is, according to clinical users, now proven to save time and improve workflow.
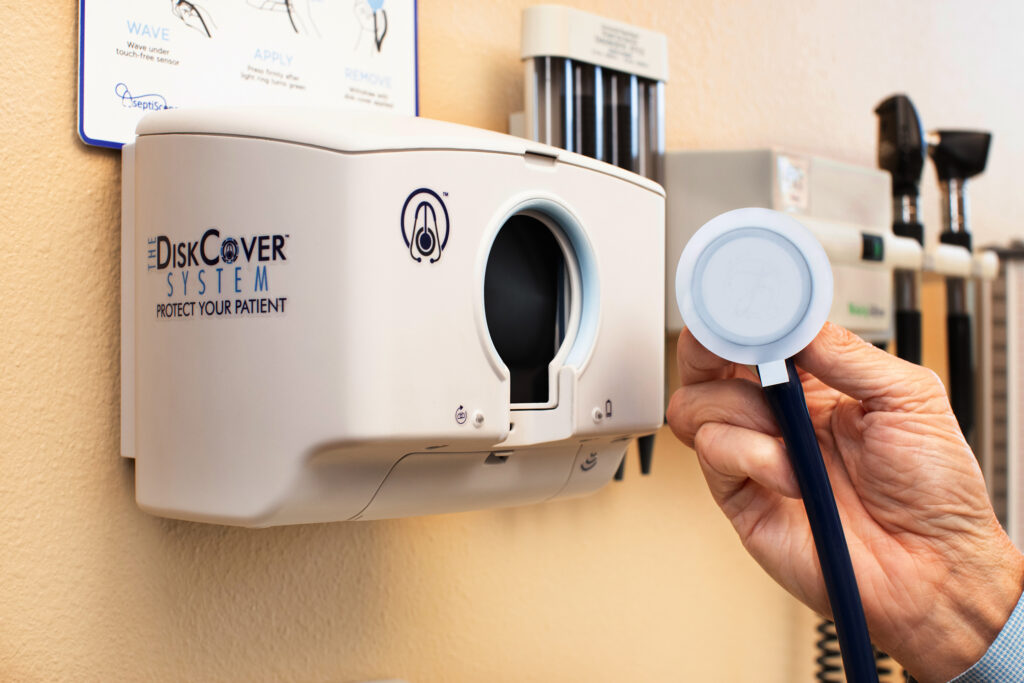
The participating centers range from primary care clinics in New York City to the intensive care unit at a prominent California cancer hospital. Dr. Francesca Torriani is Professor of Medicine in the Division of Infectious Diseases and Global Health and also the Program Director for Infection Prevention and Clinical Epidemiology at the University of California, San Diego, where she and her colleagues have been researching the impact of The DiskCover System from AseptiScope. "We have established that this technology effectively breaks transmission of all contaminants and pathogens, including alcohol resistant bacteria," Torriani stated. "Our urgent care team utilizes the product in their clinic and perceives this technology to be easy to use while advancing patient safety." In addition, Dr. Torriani remarked that switching to this touch free technology allows for the safe use of the provider's professional stethoscope, thus providing a high-quality auscultation, instead of disposable stethoscopes that have been proven to be unreliable. Furthermore, compared to disposable stethoscopes, The DiskCover System will decrease hospital waste and thus increase overall sustainability.
"We have known that the stethoscope is an efficient vector for transmission of pathogens from patient to patient," states AseptiScope co-founder Frank Peacock, MD. "The challenge was that we have had neither an immediate nor effective means to address the problem. We developed The DiskCover System to resolve this problem once and for all. Institutions integrating The DiskCover System will save time, money, and patients can visibly witness they are protected, thus offering them comfort," Dr. Peacock concludes.
AseptiScope, Inc. (www.aseptiscope.com is a privately funded San Diego, California-based, clinical innovation company.
Visit www.diskcover.com for more information and https://store.aseptiscope.com to purchase The DiskCover System directly.
AseptiScope and related logos are registered trademarks of AseptiScope, Inc. DiskCover and related logos are trademarks of AseptiScope, Inc.
Media Contact:
AseptiScope, Inc.
Scott Mader, CEO
619-743-4088
smader@aseptiscope.com
SOURCE AseptiScope, Inc.
